Story at a glance
- All tests can potentially result in false positives and false negatives.
- Each test can be studied for how often we can expect false positives and false negatives.
- The CDC has shared guidelines for how to interpret test results.
Testing for the coronavirus is still not where it should be in the U.S., but even with the tests we have there are concerns about accuracy of the results from some tests.
One thing to understand about a test is that the result isn’t a be-all and end-all, and tests are not created equal. Because of how tests work, there’s a chance that the results given don’t match reality. The chances of that happening are usually low, but nonzero. Depending on the test, there could be a handful of people for every hundred tested that get a result that doesn’t accurately predict whether they have the disease or not.
What are true positives and true negatives?
First, you should know that a true positive result means that the test did find what it was looking for. For example, if you get tested for flu, a true positive result means that the test detected the presence of genetic material from the flu virus. On the other hand, a true false result means that no genetic material from the flu was detected.
Our country is in a historic fight. Add Changing America to your Facebook or Twitter feed to stay on top of the news.
But the trouble with tests is that you can’t be sure what you are getting is a true positive or true negative. You have to rely on probabilities. More on that later.
What are false positives and false negatives?
A false positive means that the test shows a positive result, but in reality it should be a negative result. This means that a patient may be told that they have COVID-19, but they actually do not.
A false negative means that the test shows a negative result, but it should have been a positive result. This means that the patient may be told they don’t have COVID-19, but they actually are infected.
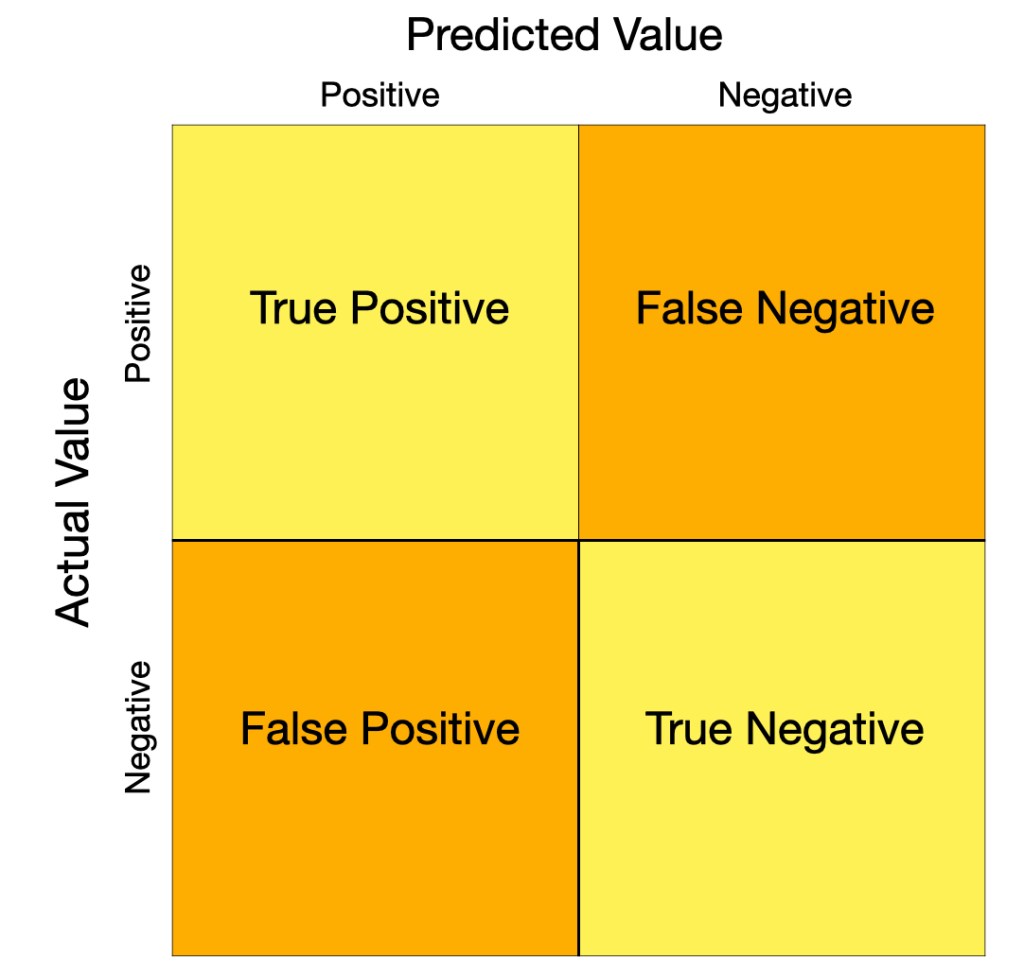
This is a confusion matrix. Aptly named, it shows the predicted values and the actual values and where they intersect to give us true and false results. Predicted values are what the test results tell you. Actual values are what they are in real life. When these don’t match up, you get false positives and false negatives. For any test, there will be a rate for how often you can expect false positives, and separately a rate for false negatives.
Why do we get false anything?
This can happen for a number of reasons. For one thing, the sample could have been contaminated with the SARS-CoV-2 coronavirus, leading to a false positive. There’s also the human aspect: a human has to process the sample to varying levels of involvement depending on how much is automated, and so the process is subject to human error.
But let’s assume that hasn’t happened. Regarding the tests, it comes down to sensitivity and specificity. And this depends on how the test works.
Specificity is how specialized the test is to the virus you are looking for. If several types of viruses share parts of their genetic sequences, what part of the genetic sequence you target will determine how specific to the virus your test is. Tests using reverse-transcription polymerase chain reaction (RT-PCR) techniques have high specificity because they are looking for a certain genetic sequence that identifies the SARS-CoV-2 virus.
The sensitivity of the test is how likely the technique will pick up a signal from the sample and depends on how much viral genetic material is present in the sample. PCR involves a step where the amount of genetic material is “amplified” or copied. This step can be done multiple times so that if there’s only a small amount of genetic material, there will be more present after each amplification step. This also means that if the sample was collected too early or too late, there’s a chance the test won’t pick up any signal that the virus is present even if it is because the volume of the virus is too low.
WHAT YOU NEED TO KNOW ABOUT CORONAVIRUS IN AMERICA
HERE’S WHEN IT’S SAFE FOR YOUR STATE TO REOPEN
HERE ARE THE 6 WAYS THE CORONAVIRUS PANDEMIC COULD END
MORE THAN 120 ATLANTA RESTAURANTS REFUSE TO OPEN DESPITE GOV KEMP LOOSENING RESTRICTIONS
CORONAVIRUS HAS MUTATED INTO MORE THAN 30 STRAINS, NEW STUDY FINDS
EXPERTS: 90% OF CORONAVIRUS DEATHS COULD HAVE BEEN AVOIDED
Sensitivity is a measure of accuracy. It’s how well the technique can identify a positive sample, how sensitive it is to finding the virus depending on how much virus is present. It’s a measure that your positive results are actual positive results. As put by biologist Maureen Ferran at the Rochester Institute of Technology for The Conversation: “A sensitive test will correctly identify people with the disease. Sensitivity measures correct positive results….A specific test will accurately identify people without the disease. Specificity measures correct negatives.”
“If a test is 90% sensitive, it will correctly identify 90% of people who are infected — called a true positive,” writes Ferran. “However, 10% of people who are infected and tested would get a false negative result – they have the virus, but the test said they don’t.”
“If a test is 90% specific, it will correctly identify 90% of people who are not infected – registering a true negative,” writes Ferran. “However, 10% of people who are not infected will test positive for the virus and receive a false positive.”
Sensitivity may be affected by the type of sample and how it was collected. In this study from Yale, experts compared nasopharyngeal swabs to self-collected saliva samples, suggesting that saliva samples may have “greater detection sensitivity and consistency throughout the course of infection.” This is a preprint on the medRxiv server, which is meant for disseminating studies among researchers before they are officially published. The study has not yet been peer reviewed, which is the process for vetting research and publishing it in an academic journal.
For any test, you can estimate the probability that a positive result represents a true positive, and a negative result represents a true negative. In order to get an estimate, the test needs to be done for a large number of samples and validated multiple times. Companies that develop tests can estimate the rate at which we can expect false positives and false negatives. These can inform us on what’s the likelihood that the result we’re given is the actual value. For all of these reasons, developing tests is more nuanced that it may seem from the outside.
Tests for the coronavirus
Diagnostic tests, or viral tests as the term used by the Centers for Disease Control and Prevention (CDC), are the kind that are used to test someone who is suspected to be sick with the coronavirus. That means they may be showing symptoms or they may have been exposed to someone who was confirmed to have COVID-19.
Most diagnostic tests for the coronavirus work by detecting any fragments of the virus’s genetic material. For example, the CDC Real-Time RT-PCR diagnostic panel targets regions of the virus nucleocapsid gene that codes for the outer shell of the virus. The ID NOW machine does a rapid test for SARS-CoV-2 by targeting the RdRp gene, which codes for an RNA polymerase that is part of the mechanism that replicates the virus, according to its website. The McGovern Institute at the Massachusetts Institute for Technology (MIT) and the Broad Institute are developing a test based on CRISPR technology, which is used worldwide by researchers who do gene editing in their labs.
Antibody tests aren’t looking for the coronavirus itself in an active infection, but the antibodies produced by the immune system that are specific to the virus. Antibodies are proteins that can attach to foreign particles in the body like a virus by targeting a specific protein on the outside of the virus. An antibody test is most appropriate for people who suspect they had COVID-19 and are now recovered.
There’s a range of specificity. One preprint study posted on medRxiv that has yet to be peer-reviewed found that specificity of antibody tests can range from 84.3 to 100.0 percent. This means that some tests may result in 15 false negatives for every 100 tests, while others result in fewer.
What we know about COVID-19 tests
There are a number of COVID-19 diagnostic and antibody tests available. Here’s what we know about them in terms of false positives and false negatives.
Diagnostic tests have a range of success in detecting virus in positive samples. A test called DiaSorin Simplexa detected 89.3 percent of infections in a study, reports NPR. A test by Roche detected 96.5 percent of positive samples and a test from Cepheid detected 98.2 percent of infected samples, Gary Procop tells NPR.
Five tests that were studied by a nonprofit in Germany were found to have 100 percent sensitivity on positive samples and 96 percent specificity on negative samples. However, this study was conducted in ideal settings, which may be far from real world conditions.
ID NOW, the countertop machine that health professionals use for testing for flu and strep, may miss about half of the patients who have low levels of virus, according to reporting by NPR. Overall, it detects the coronavirus in 85.2 percent of positive samples, meaning it has a false negative rate of 14.8 percent.
Quest Diagnostics has an antibody test that it reports has specificity of 99 to 100 percent, meaning it has 1 percent or less false positives. The test also has a “clinical performance of approximately 90 percent to 100 percent (assessed as percent agreement of serology results on known COVID-19 PCR positive cases),” according to its website. This means there could be up to 10 percent false negatives. To note, getting a positive result from an antibody test does not mean the patient has immunity. The amount of detectable antibody in the blood may decrease over time after the infection has passed.
If all of this still feels confusing and daunting, the CDC has made a chart with guidelines on how to interpret test results.
For up-to-date information about COVID-19, check the websites of the Centers for Disease Control and Prevention and the World Health Organization. For updated global case counts, check this page maintained by Johns Hopkins University.
You can follow Chia-Yi Hou on Twitter.
BREAKING NEWS ABOUT THE CORONAVIRUS PANDEMIC
WHO: THERE’S NO EVIDENCE WEARING A MASK WILL PROTECT YOU FROM CORONAVIRUS
HERE’S WHEN THE CORONAVIRUS WILL PEAK IN YOUR STATE
FAUCI PREDICTS ANOTHER CORONAVIRUS OUTBREAK IN THE FALL WITH A ‘VERY DIFFERENT’ OUTCOME
BILL GATES SEES RNA VACCINES AS BEST OPTION FOR QUICK CORONAVIRUS TREATMENT
NEW REPORT SAYS CORONAVIRUS PANDEMIC COULD LAST UP TO 2 YEARS
changing america copyright.
